The researchers from Prof. ZHONG Jin's group at the Institut Pasteur of Shanghai of the Chinese Academy of Sciences and Prof. WEI Wensheng’s group at Peking University as well as Dr. ZHANG Guigen’s group at Sun Yat-sen University reported that TRIM26 is a critical host factor for HCV replication and contributes to host tropism. The study was published online in Science Advances on January 8, 2021.
Hepatitis C virus (HCV) is an RNA virus belonging to the Hepacivirus genus of the Flaviviridae family and is an important pathogen that causes hepatitis C. HCV has evolved a variety of strategies to escape host immune system to establish chronic infection. Long-term chronic infection can lead to severe live diseases such as cirrhosis and liver cancer. Direct-acting antiviral agents (DAA) has greatly improved the efficiency of hepatitis C treatment, but due to the lack of vaccines, the eradication of HCV is still very challenging. Animal models of HCV infection are crucial tools for the development of hepatitis C vaccine.
HCV only infects humans and chimpanzees, but due to ethical issues, the use of chimpanzees as model animals is now prohibited. Transgenic mice expressing HCV receptors can support HCV infection to a limited extent. However, due to the lack of other human host factors, especially the ones essential for the steps after the virus enters the cell, the application of this transgenic mouse model in evaluating the protection efficacy of HCV vaccines remains limited. Therefore, the identification of new species-specific HCV host factors is of great significance for the establishment of HCV small animal infection models.
To identify essential host factors in HCV infection, the researchers used the previously established HCV live cell real-time reporting system to perform genome-wide CRISPR/Cas9 screening and found for the first time that TRIM26, an E3 ubiquitination ligase, is an important host factor of HCV. The result showed that TRIM26 specifically promotes the replication of the HCV genome, but has no effect on the replication of other viruses belonging to the same Flaviviridae family (such as Dengue virus and Zika virus).
To study how TRIM26 promoted HCV genome replication, the researchers examined the interaction between TRIM26 and HCV proteins. The results demonstrated that TRIM26 interacts with virus-encoded NS5B protein, an RNA-dependent RNA polymerase, to catalyze the K27-linked ubiquitination modification of lysine 51 in NS5B, thereby enhancing the interaction between NS5B and NS5A, another key component of the virus replication complex, and ultimately promoting the replication of the HCV genome.
Furthermore, the researchers also compared the effects of TRIM26 from different host species on HCV replication, and found that TRIM26 from humans and tree shrews can support virus replication, whereas TRIM26 from mice cannot do so. Amino acids analysis revealed that compared with other species, mouse TRIM26 has a unique 6-amino acid insertion. The mouse TRIM26 with a deletion of this insertion can partially restore its interaction with the HCV NS5B and its ability to enhance virus genome replication. Finally, the researchers found that the ectopic expression of human TRIM26 in mouse hepatic cells can significantly enhance HCV infection.
Overall, this study not only discovered a novel HCV host factor and further deciphered the molecular mechanism of HCV replication, but also provided a new strategy to develop a small animal model for HCV infection.
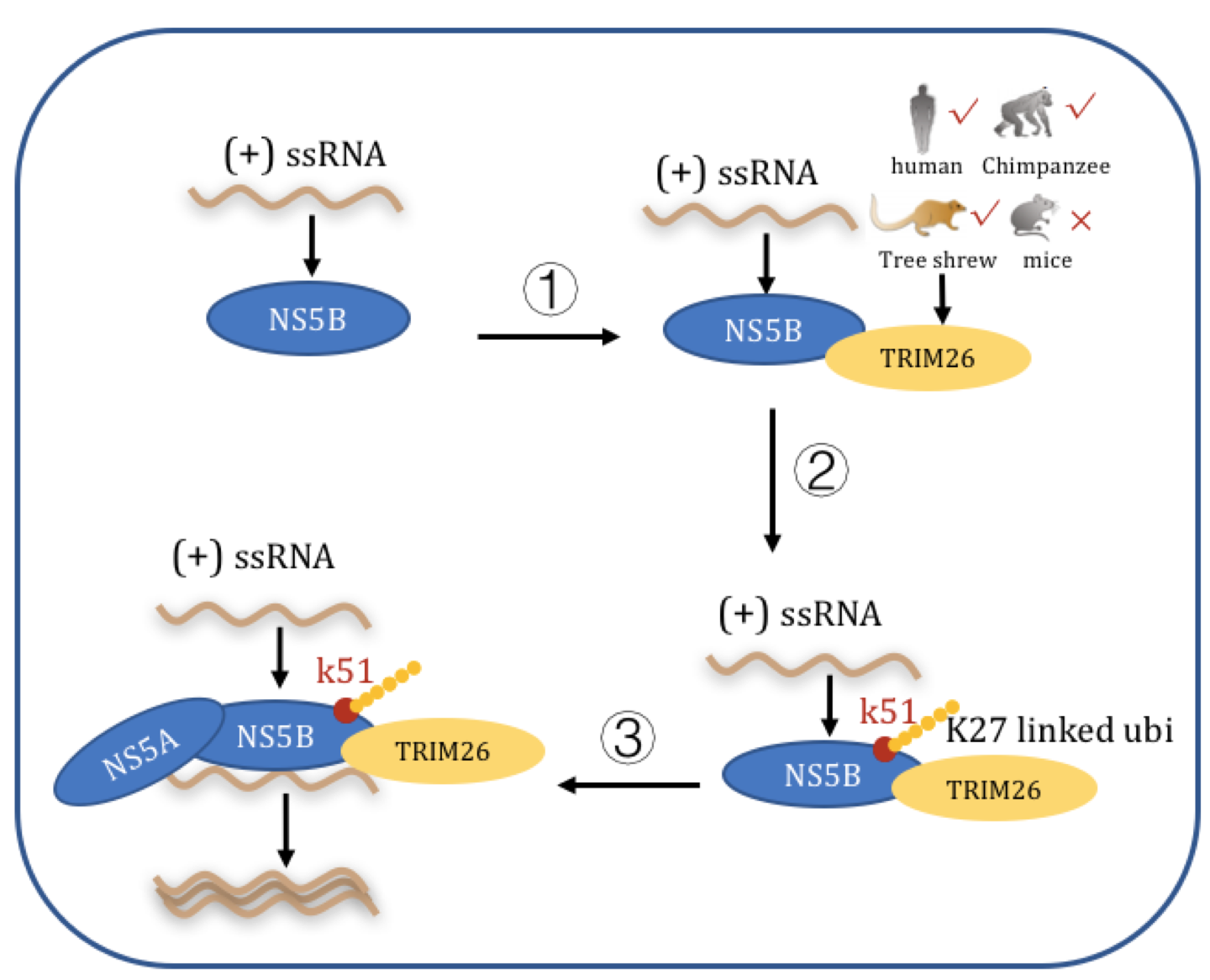
Working model of TRIM26 (from human and tree tupaia) enhancing HCV genome replication. (Image by IPS)
Article link: https://advances.sciencemag.org/content/7/2/eabd9732

