Hepatitis C virus (HCV) is one of the major pathogens which will cause chronic hepatitis, cirrhosis, and hepatocelluar carcinoma. A protective vaccine is not yet available and the standard treatment with PEG-interferon alpha plus ribavirin usually takes more than one year and has strong side effect, and only 40%-60% patients can be finally cured. Therefore, new generation of therapy focuses on small molecules which can target specific viral proteins.
p7 is a viroporin protein encoded by HCV, which plays a crucial role during the virus assembly and release. As a small, hydrophobic membrane protein, p7 is hard to be crystallized. In this case, people had no information about the detail structure of p7 and thus little progresses had been made in developing anti-HCV drugs targeting p7.
Recently, Prof. James Chou and his colleagues have determined the high resolution structure of p7 viroporin using the latest nuclear magnetic resonance (NMR) technologies. The structure exhibits an unusual mode of hexameric, flower-like assembly. Besides, Prof. SUN Bing’s lab used their established viral channel platform to exam the p7 channel activity and drug inhibition characters. p7 was expressed in oocytes from Xenopus Laevis and the membrane conductance was tested using a two electrode voltage clamp (TEVC). This functional investigation identified key residues that are important for channel activity implicated by the structure model. In summary, this new structure can help us better understand the important role of p7 in the virus life cycle, and shed light on new anti-HCV small molecule drugs.
This research was published online in Nature on 5th June. And it’s conducted by Prof. James Chou’s group from Harvard Medical School and Shanghai Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (SIBCB-SIBS-CAS), in collaboration with Prof. SUN Bing from Institut Pasteur of Shanghai, CAS and SIBCB-SIBS-CAS.
This work was supported by the National Key Project of 973 (2013CB530504) and National Science and Technology Major Project (2012ZX10002-007-003) (to SUN Bing) and NIH grant GM094608 (to James Chou).
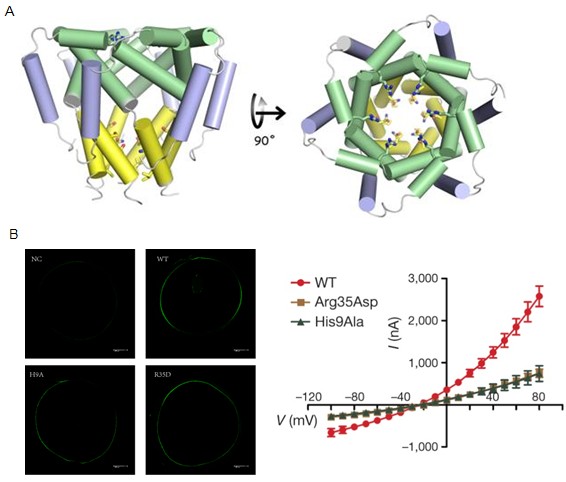
A.NMR structure of the p7 hexamer
B. Validation of the Arg35 and His9 as important residues for channel activity

