A joint research team led by Drs. Zhong Huang and Xia Jin at the Institut Pasteur of Shanghai (IPS), ChineseAcademy of Sciences (CAS), has recently completed preclinical studies of recombinant protein-based candidate Zika vaccines. This work was published as a research article entitled “Insect cell-produced recombinant protein subunit vaccines protect against Zika virus infection” in the journal Antiviral Research on April 19, 2018.
Zika virus (ZIKV) is an RNA virus belonging to the flavivirus genus of the Flaviviridae family. The virus is mainly transmitted by mosquitoes. In the past several years, large ZIKV outbreaks have occurred in the Pacific regions and the Americas, with millions of people infected. Clinical studies have revealed associations between ZIKV infection and neurological diseases such as microcephaly and Guillain-Barré syndrome. The ability of ZIKV to cause microcephaly and other birth defects has been confirmed in mice and non-human primates. Clearly, ZIKV is now posing a serious threat to global public health. However, no commercial vaccine is available to prevent ZIKV infection.
To develop recombinant vaccines for ZIKV, the IPS research team had produced different forms of ZIKV envelope protein (E) in the Drosophila S2 insect cell expression system, and subsequently evaluated their immunogenicity and protective efficacy in mice. The results showed that the N-terminal approximately 80% region (designated as E80) and the domain III (designated as EDIII) of ZIKV E protein could be efficiently produced as secreted proteins in the S2 cell expression system. Both E80 and EDIII elicited antigen-specific neutralizing antibody and T-cell responses in mice. Importantly, passive transfer of either anti-E80 or anti-EDIII sera protected recipient mice against lethal ZIKV challenge. It is worth noting that, at the same dose, EDIII induced higher neutralizing antibody titers than did E80, and the resulting anti-EDIII sera appeared to confer better protection against experimental ZIKV infection compared to the anti-E80 sera. These data indicate that the S2 cell-produced EDIII represents an elite recombinant Zika vaccine candidate worthy of further preclinical development and clinical testing.
This work is supported by grants from the National Major R&D Program of China, grants from the ChineseAcademy of Sciences, and a grant (ZIKAlliance) from the EU Horizon 2020 program.
Links:https://doi.org/10.1016/j.antiviral.2018.04.010
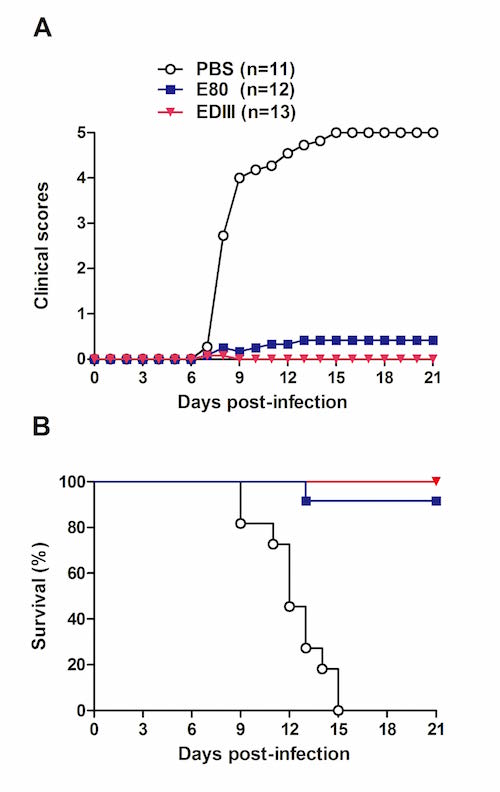
In vivo protective efficacy of the recombinant vaccines

