Malaria is an infectious disease that presents serious threat to human life and health. Among the human malaria parasites, Plasmodium falciparum is the deadliest, causing millions of clinical infections worldwide every year. The surface variant antigen P. falciparum Erythrocyte Membrane Protein 1 (PfEMP1), encoded by ~60 var genes, mediates binding of parasitized erythrocytes to endothelial surface and contributes to the major pathogenesis in severe malaria. Mutually exclusive expression of the var gene family contributes to antigenic variation and immune evasion in P. falciparum. Elucidation of the expression mechanism of var gene family can provide new targets for the development of malaria vaccine and antimalarial drugs. So this research has always been the international hotspot of this field.
JIANG Lubin's team at the Institut Pasteur of Shanghai, Chinese Academy of Sciences (IPS, CAS) has ever demonstrated that histone methylase PfSET2 (Nature, 2013) and a class of long non-coding RNA (Front Microbiol, 2018) are involved in regulating the silencing and activation of var gene, respectively. However, the traditional gene editing technology of Plasmodium falciparum is time-consuming and very inefficient, which hinders the understanding of the entire var gene regulatory network. Therefore, other relevant regulatory factors are still unclear. Using the epigenetic gene editing technology of Plasmodium falciparum based on CRISPR/dCas9 system developed by Professor Jiang's team, this work discovers that the entire var gene family goes silenced upon knockout of the PfRecQ1 DNA helicase, and the clonal expression of the var genes could be rescued by the PfRecQ1 complementation. Additionally, their study shows that PfRecQ1 maintains var gene clonal expression possibly by mediating the nuclear periphery localization of the var gene family and decreasing heterochromatic histone modifications at the var gene loci. This work reveals a new path of var gene regulation through chromatin structure and identifies PfRecQ1 as a novel target for antimalarial drug development.
Graduate students LI Zhou, YIN Shigang, SUN Maoxin and CHENG Xiu from IPS, CAS are the first authors of this paper. Professor JIANG Lubin, DAI Xueyu and HUANG Zhenghui from IPS, CAS are the correspondence author. This work was supported by grants from the National Key R&D Program of China, National Science and Technology Major Project, National Natural Science Foundation of China, and National Institute of Allergy and Infectious Diseases Grants.
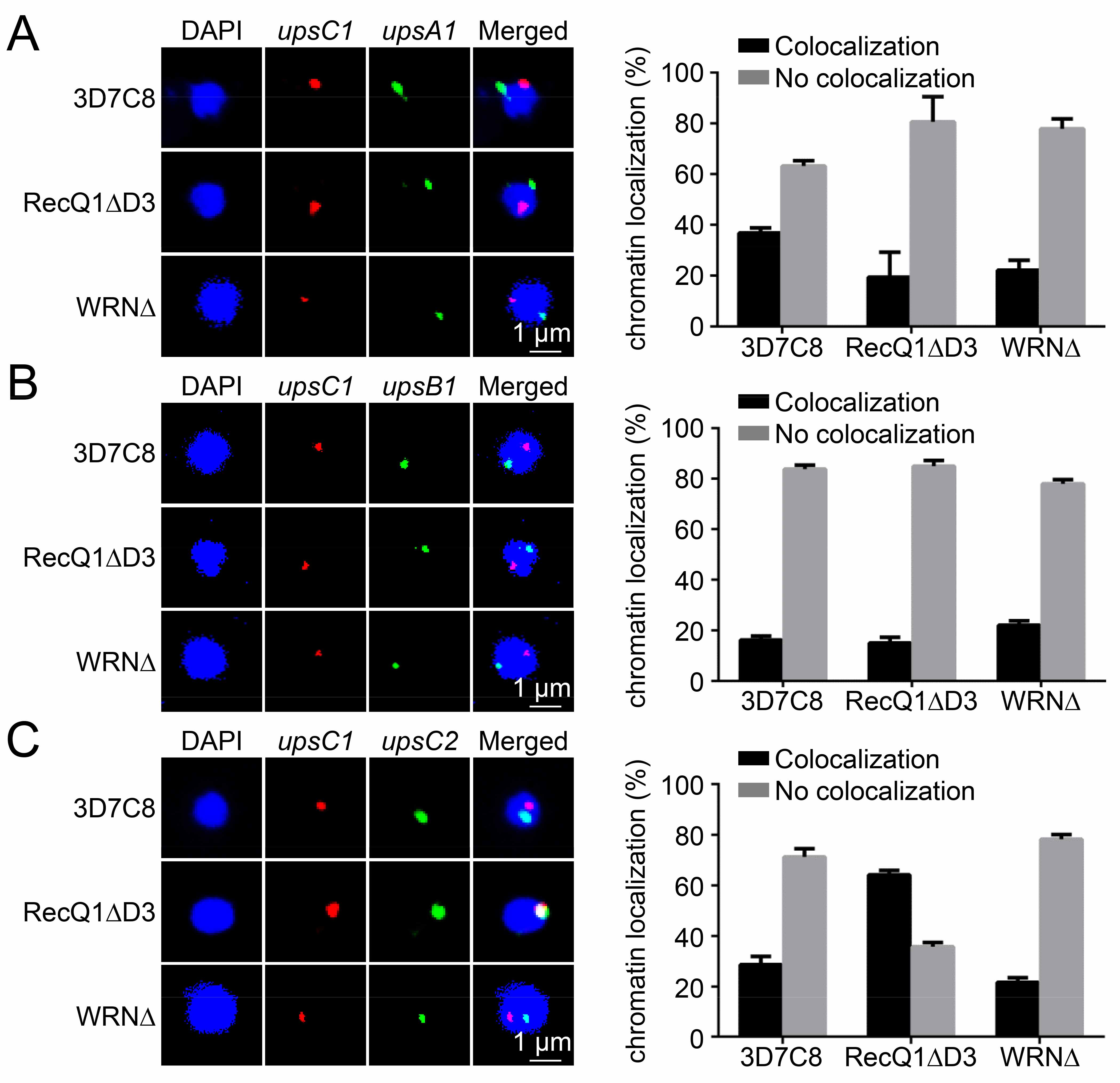
Figure: Var gene DNA redistribution correlates with its expression status upon PfRecQ1 deletion.
Publication onlined: https://www.pnas.org/content/early/2019/02/05/1811766116

