The research paper entitled “Cryo-EM structures of Smc5/6 in multiple states reveal its assembly and functional mechanisms” was published online in Nature Structural & Molecular Biology (NSMB) on June 18, 2024. This work was carried out by the collaborative teams of Prof. WANG Lanfeng at the Shanghai Institute of Immunity and Infection of the Chinese Academy of Sciences (CAS), Prof. CHEN Zhenguo at Fudan University, and Prof. ZHAO Xiaolan at the Memorial Sloan Kettering Cancer Center. 
The Structural Maintenance of Chromosome (SMC) complex plays a crucial role in the highly ordered and dynamic assembly of eukaryotic genomes into chromatin, which further condenses into chromosomes as carriers of genetic information. The SMC family primarily consists of three members: cohesin, condensin, and the Smc5/6 complex in eukaryotes. Initially identified for its role in maintaining genome stability and DNA damage repair, the Smc5/6 complex has been recently revealed to have significant roles in restricting virus episome replication/transcription processes, potentially making it a novel target for antiviral therapy. The study deived into the assembly and functional mechanisms of this multi-subunit protein machinery.
The Smc5/6 complex comprises eight subunits of Smc5, Smc6, and six non-SMC elements (Nse1-6). However, despite developing a general knowledge of the significance of these subunits, the lack of structural information on the intact Smc5/6 complex hinders the understanding of its diverse biological functions.
The researchers prepared homogeneous protein complex samples using the baculovirus insect cell expression system. Using cryo-EM technology, they solved the three-dimensional structures of the Smc5/6 complex of Saccharomyces cerevisiae in three different states (8-subunit intact Smc5/6 complex (-8mer), 5-subunit complex lacking Nse1-3-4 (-5mer), and 6-subunit complex lacking Nse5-6 (-6mer)). Among them, the resolution of the 8-subunit Smc5/6 complex is up to 3.2 angstroms, providing a detailed structural basis to elucidate the complex's composition mode and functional mechanism at the atomic level (Fig.1).
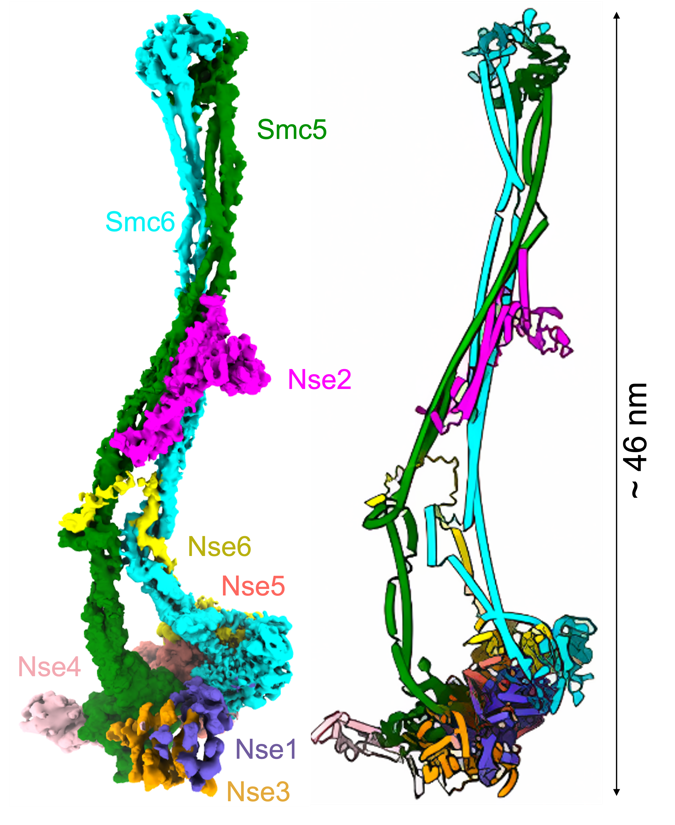
Fig. 1 Cryo-EM structure of 8-subunit Smc5/6 complex. (Image by SIII)
The researchers found that due to the unique structural properties of the Nse2 subunit and Smc5, the complex forms a "long rod" structure with a length of ~46 nm, which is different from the "arm-folded" structure of other members of the SMC family. In addition, they revealed that the Nse2 subunit contains a "wedge" motif formed by a ~3-turn helix and a "hook" motif at the N-terminus of the Nse6 subunit. By bridging the Sm5-Smc6 heterodimers, these two structural motifs play a crucial role in maintaining the stability of the entire complex and its function in DNA repair.
Through structural analysis in various states, the researchers found that the structure of the Nse1-3-4 and Nse5-6 subcomplexes are highly conserved, indicating that the composite assembly process may be implemented in a modular manner. At the same time, the molecular mechanisms of the two subcomplexes Nse1-3-4 and Nse5-6, which antagonistically regulate the ATPase activity of the Smc5/6 complex, were structurally elucidated.
Based on the ATPase activity, hypotheses can be made that the Smc5/6-8mer intact complex represented a "semi-inhibitory" state, the -5mer complex missing Nse1-3-4 was an inactive intermediate state, and that the -6mer showed a more active state once the Nse5-6 dissociated from the -8mer complex (Fig. 2). This regulatory mechanism is not common in other complexes of the SMC family, suggesting that the Smc5/6 complex has a unique regulatory mechanism during DNA manipulation.
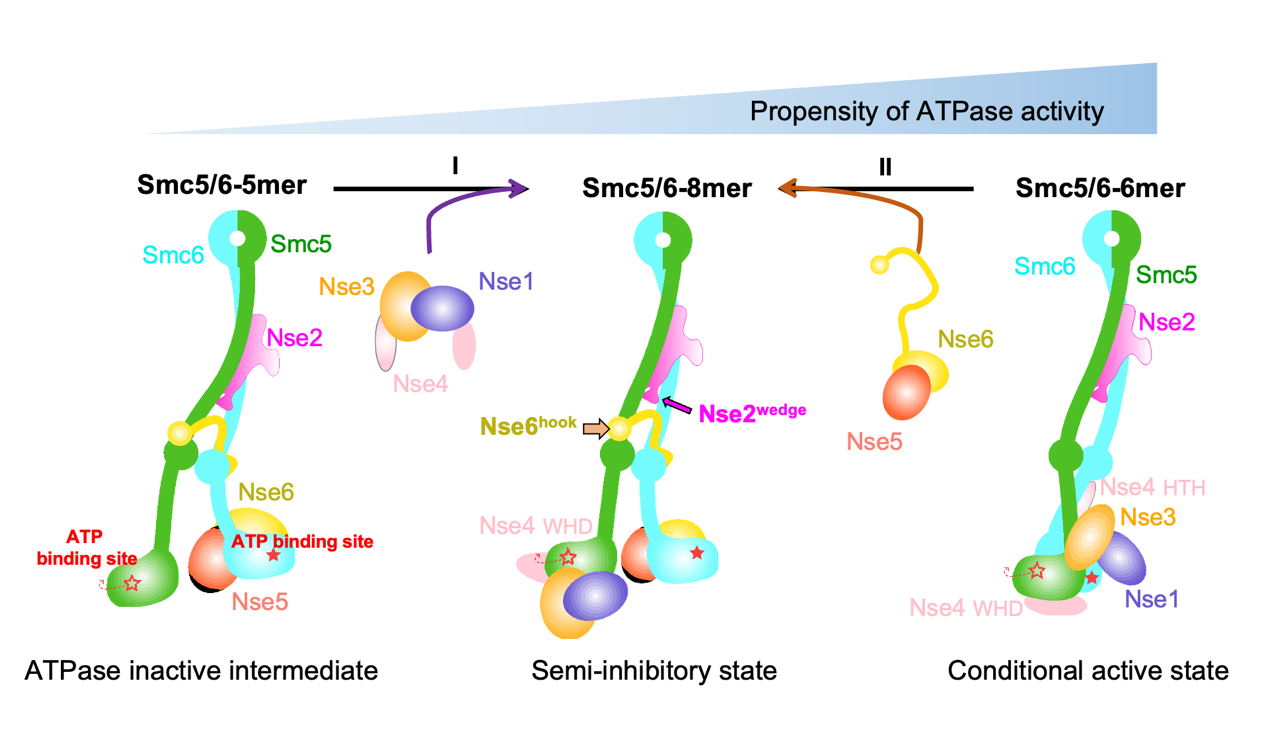
Fig. 2 A model of structural changes and associated ATPase activities of three Smc5/6 complexes. (Image by SIII)
The results of this study serve as a vital structural basis for an in-depth understanding of the functional mechanism of the Smc5/6 complex, demonstrating its great significance for further exploring its potential roles in the occurrence and development of related diseases.
Contact:
WANG Lanfeng
Shanghai Institute of Immunity and Infection, CAS
Reference:https://www.nature.com/articles/s41594-024-01319-1

