On May 20, 2025, a research team led by Prof. CHEN Changbin at the Shanghai Institute of Immunity and Infection of the Chinese Academy of Sciences, published a study titled “Targeting epigenetic regulators to overcome drug resistance in the emerging human fungal pathogen Candida auris” in Nature Communications. Working with Prof. HUANG Xiaotian from School of Basic Medical Sciences, Jiangxi Medical College, Nanchang University and Prof. ZHOU Dongsheng from State Key Laboratory of Pathogen and Biosecurity, Academy of Military Medical Sciences, the team unveiled a promising new approach to counter the growing threat of drug-resistant Candida auris. Their work pinpoints a key epigenetic regulator, Gcn5, as a potential target to reverse antifungal resistance and improve treatment outcomes.
First identified in 2009, C. auris has rapidly emerged as a global public health concern, spreading to over 40 countries and causing severe outbreaks in healthcare settings, especially in intensive care units. This fungal pathogen is notoriously difficult to treat due to its high resistance to multiple antifungal drugs - fluconazole resistance exceeds 90%, and it can quickly evolve new resistances. The mortality rate of invasive C. auris infections can reach up to 70%, and its ability to persist on hospital surfaces and resist disinfection significantly increases the risk of transmission. In recognition of these dangers, the World Health Organization (WHO) has listed C. auris as a top-priority fungal pathogen.
Chemical modifications to histones play a key role in regulating gene activity and have been extensively studied in cancer research. Changes such as histone acetylation and methylation can influence how cancer cells grow and spread, leading to the development of therapies that target these processes, including HDAC inhibitors. However, their application in infectious disease research is still in its early stages. This study investigated how post-translational modifications of histone H3 affect the resistance and virulence of C. auris.
The researchers conducted a comprehensive analysis of histone H3 modifiers, focusing on acetyltransferases and methyltransferases. They discovered that Gcn5, a histone acetyltransferase, plays a central role in C. auris drug resistance. GCN5 depletion and the subsequent loss of histone H3 acetylation downregulates key genes involved in ergosterol biosynthesis and drug efflux, resulting in increased susceptibility to azoles and polyenes. Additionally, Gcn5 regulates cell wall integrity and echinocandin resistance through the calcineurin signaling pathway and transcription factor Cas5. In infection models using Galleria mellonella and immunocompromised mice, GCN5 deletion significantly reduces the virulence of C. auris.
To test its therapeutic potential, the team used CPTH2, a compound that inhibits Gcn5 activity. When combined with the antifungal drug caspofungin, CPTH2 significantly boosted antifungal efficacy in vitro and in vivo, without detectable toxicity to human cells or mice.
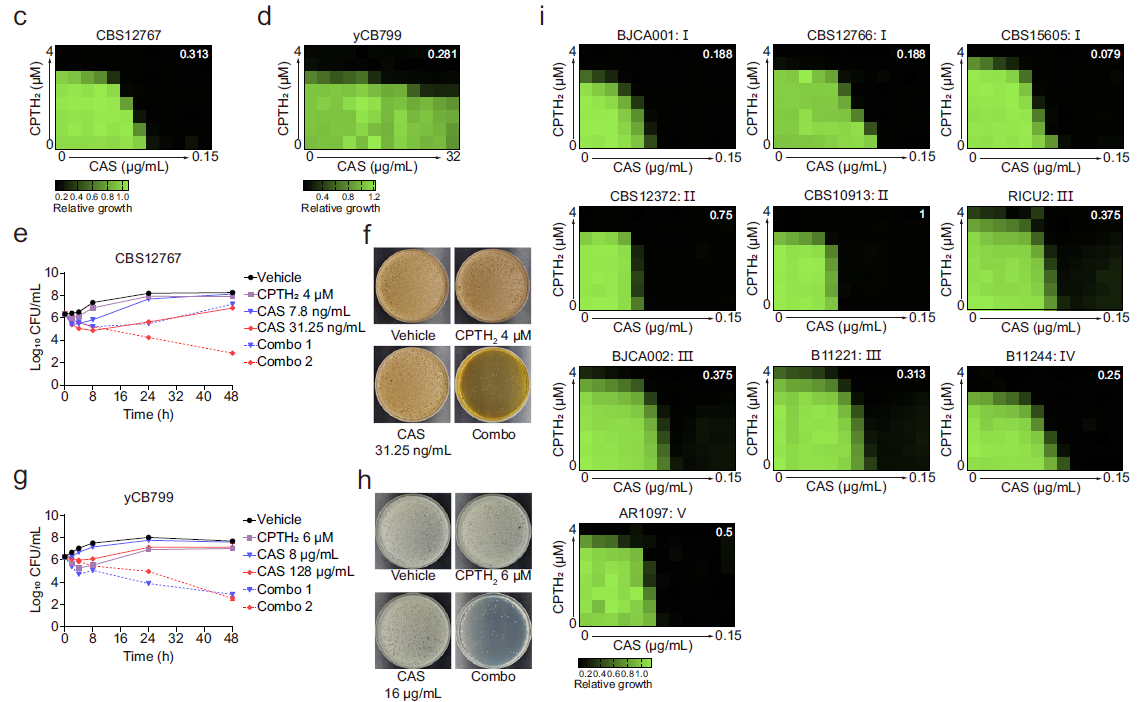
Figure 1. Synergistic fungicidal activity of Gcn5 inhibitor CPTH2 combined with caspofungin. (Image by SIII)

Figure 2. In vivo safety profile of CPTH2 demonstrates good biocompatibility in mouse models. (Image by SIII)
This study is the first to identify Gcn5 as a key regulator of both drug resistance and pathogenicity in C. auris. By targeting this epigenetic regulator, the researchers propose a new antifungal strategy to address the urgent challenge of multidrug resistance. As C. auris continues to spread globally and exhibit high levels of treatment resistance, further refinement of CPTH2 or the development of more potent Gcn5-specific inhibitors could lead to effective new therapies for this life-threatening pathogen.
Contact:
CHEN Changbin
Shanghai Institute of Immunity and Infection, Chinese Academy of Scineces
cbchen@siii.cas.cn
Reference: https://www.nature.com/articles/s41467-025-59898-6

