Radiotherapy and chemotherapy are widely practiced clinical applications with the ability to shape the tumor immune microenvironment,in which the cGAS-cGAMP-STING pathway plays a crucial role in augmenting anticancer immunity.
Studies have suggested that transferring cGAMP from tumor cells to infiltrating immune cells is a unique way to overcome the tumor cells’ lack of STING expression and elicit a robust type I interferon response. However, the precise mechanism underlying cGAMP’s cell-to-cell transfer within the tumor environment remains poorly understood.
In a study published in Science Immunology on Jun 27, Prof. XIAO Hui’s team at the Institute of the Chinese Academy of Sciences, and Prof. LI Huabin’s team from the Affiliated Eye and ENT Hospital of Fudan University, unveiled a novel mechanism by which LRRC8/VRAC transports tumor-derived cGAMP into infiltrating immune cells to enhance the cytolytic functions of CD8 T cells and natural killer (NK) cells during radiotherapy and chemotherapy, providing new insight into the improvement of the conventional cancer therapies.
Prof. XIAO’s team has been focusing on deciphering the complex innate immune mechanisms against pathogens and cancers. The team has identified the Volume-Regulated Anion Channel LRRC8/VRAC as a bona fide cGAMP transporter, promoting the type I interferon response that is crucial for containing infections by herpes viruses, such as HSV-1.
In this study, the researchers revealed that the LRRC8A/C-containing VRAC channels play a vital role in mounting the anticancer immune responses to radiotherapy and chemotherapy. LRRC8/VRAC transfers cGAMP from irradiated cancer cells to infiltrating CD4 and CD8 T cells, thereby inducing a prominent type I interferon response that boosts the cytolytic effector functions of CD8 T cells and natural killer cells.
Moreover, the researchers demonstrate that T-cell receptor (TCR) activation by the cognate tumor antigen serves as a physiological signal to open the VRAC pore through PI(4,5)P2 and reactive oxygen species (ROS), allowing the rapid uptake of cGAMP and STING activation in mouse and human T cells. Additionally, tumor-derived extracellular ATP can act in synergy with TCR signaling to accelerate the transfer of extracellular cGAMP, which is sensitive to ENPP1 degradation.
This study further suggests that simultaneously inhibiting the extracellular ATP hydroxylase CD39 and the cGAMP hydroxylase ENPP1 can significantly enhance LRRC8/VRAC-mediated cGAMP transfer, leading to superior anticancer immunity.
“It is remarkable that we identify LRRC8/VRAC as a bona fide cGAMP transporter pivoting radiotherapy and chemotherapy’s anticancer efficacy. We believe that this finding could open up new avenues for future cancer immunotherapy bolstering T cell’s LRRC8/VRAC function,” said Prof. XIAO.
This study demonstrates the simultaneous boosting of extracellular ATP and cGAMP as a novel approach to enhance the efficacy of radiotherapy, thereby providing proof-of-concept for harnessing in situ extracellular cGAMP to improve cancer therapy.
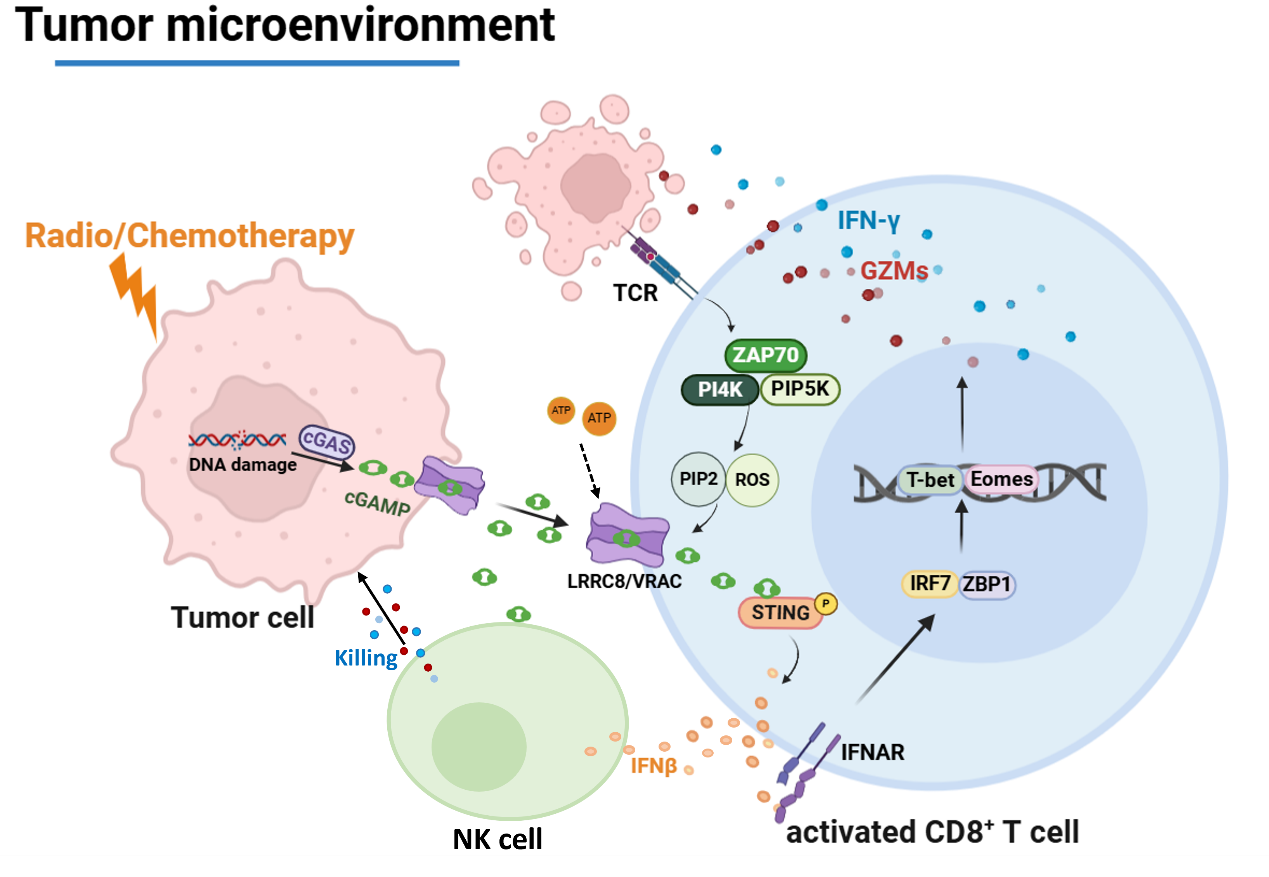
Figure. LRRC8/VRAC-mediated cGAMP transfer underlines radiotherapy and chemotherapy. (Image by SIII)
Contact:
XIAO Hui
Email: huixiao@siii.cas.cn
Reference: https://www.science.org/doi/10.1126/sciimmunol.adn1630

